|
Stereo Vision
Being able to visualize and experience strucutre in 3-D is an essential skill, if you are at all serious about understanding the molecules of molecular biology.
Even though hardware devices exist that help in the three-dimensional perception of computer graphics images, for the serious structural biologist there is really no alternative to being able to fuse stereo pair images by looking at them. RasMol is an excellent tool to practice stereo vision and develop the skill. Stereo images consist of a left-eye and a right-eye view of the same object, with a slight rotation around the vertical axis (about 5 degrees). Your brain can accurately calculate depth from these two images, if they are presented to the right and left eye separately. This means you need to look at the two images and then fuse them into a single image - this happens when the left eye looks directly at the left image and the right eye at the right image.
In fact, it can also be the other way around and some people find convergent (cross-eyed) stereo viewing easier. I recommend the divergent (wall-eyed) viewing - not only because it is much more comfortable in my experience, but also because it is the default way in which stereo images in books and manuscripts are presented.
In order to visually fuse stereo image pairs, you need to override an ocular reflex that couples divergence and focussing, this is something that needs to be practiced for a while. Usually 5 to 10 minutes of practice twice daily for a week should be quite sufficient. It is not as hard as learning to ride a bicycle, but you need to practice regularily for some time, maybe 10 or 20 sessions of 3 to 5 minute over a period of a week or two. Once you have acquired the skill, it is really very comfortable and can be done effortlessly and for extended periods. You will enter a new world of molecular wonders !
Here are step by step instructions of how to practice stereo-viewing with RasMol.
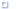 |
Load a small protein into RasMol and display this as a simple backbone model. |
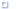 |
Type set stereo -5 in the command line (the RasMol default is cross-eyed, thus the need to specify a negative rotation angle). |
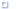 |
Resize the window, until two equivalent points on the protein are the same distance on the screen, as your eyes are apart (this is usually about 6 cm). |
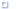 |
Touch your nose to the screen and look at the two images. They will be blurred and out of focus, but should appear as a three-dimensional object. Slowly rotating the protein helps. |
 |
Once you see the object in 3-D, try to move your head backwards slowly, until the structure comes into focus by itself. Do not voluntarily try to focus, since this will induce your eyes to converge and you will lose the 3-D effect. When you lose the 3-D effect, start over. |
 |
Practice this patiently, two times daily for some 3 to 5 minutes. Stop, when your head feels funny. Don't force yourself. It should take you about a week to master this, with regular training it will become very easy. And, the best thing is, you do not easily forget this skill. It is like riding a bicycle, equalizing pressure in your eustachian tubes while scuba diving, or circular breathing to play the didgeridoo: once you teach your body what to do, it remembers. And expands your horizon. |
Below, there is a script to set up a stereo-view of 2IMM. Simply copy the script from the textarea and paste it into the RasMol command line window. The commands get executed line by line and it is easy to change parameters and arguments and see what effect this has.
|
A set of script commands for a simple view of 2IMM, suitable to practice stereo viewing.
|
|
|
Here are some suggestions for stereo viewing mini-projects, so your practice sessions do not become monotonous. They are ordered from the most simple scenes to progressively more complicated molecules.
| 3D mini-project topics |
PDB source files |
| Study individual amino acids and memorize the spatial arrangement of the groups around the chiral centre (the C-alpha atom) in this L-amino acid. |
Alanine
Cysteine
Aspartate
Glutamate
Phenylalanine
Glycine
Histidine
Isoleucine
Lysine
Leucine
Methionine
Asparagine
Proline
Glutamine
Arginine
Serine
Threonine
Valine
Tryptophan
Tyrosine
|
| Study (and remember) the correct chirality of the Threonine side-chain. |
Threonine
|
| Download and study small molecule cofactors from HIC-UP. Some samples are linked here. |
ATP
Arachidonic acid
Beta-carotene
Biotin
Caffeine
FAD
FMN
Phycoerythrobilin
Testosterone
|
| Study an alpha-helix. Concentrate on how the carbonyls are all oriented in the same direction. Since the carbonyl carries a significant negative dipole there is a large electrostatic dipole moment induced along the alpha helix. Memorize how this arrangement relates to the N- and C-terminus of the helix. Is it the N- or the C-terminus of the helix that lies in the strongly positive potential region of the helix dipole ? Where would a negatively charged residue (such as a phosphate group) find a binding site: at the beginning or the end of a helix ? |
Helix
|
| Study a beta-sheet. Concentrate on how the alternating hydrogen bonds are formed between pairs of residuesin opposite direction. The example provided also has a cis-proline. Find it and study why the C-alpha atoms of the preceeding residue and of the proline are in cis, relative to the peptide bond ? |
Sheet
|
| Display only the backbone, and the side chain atoms of Glu, Asp (color red) and Lys, Arg (color blue) residues in a protein. Pick out the salt-bridges in your structure. |
|
| Download a DNA molecule. Study the phosphate backbone connections. Study and remember the arrangement of the 5' and 3' hydroxyl and the phosphate group. You should be able to discern the sequence of a DNA molecule from viewing the structure. You should also remember which way the helix turns: DNA is a right handed helix (except Z-DNA which is left-handed). |
B-DNA
A-DNA
Z-DNA
|
| Study the catalytic triad in a serine protease. In this structure (one of the classics: bovine trypsin in complex with the pancreatic trypsin inhibitor, by Robert Huber and Johann Deisenhofer, 1982) the triad is serine 195, histidine 57 and aspartate 102. |
2PTC.pdb
|
| Study the ATP and t-RNA binding sites in a tRNA synthetase. Note the relative sizes of nucleotide structures and proteins. Pay special attention to the way the anticodon loop is tightly bound, as well as the acceptor stem. Using stereo, it should not be difficult to pick out the AMP molecule bound in this structure of E. coli glutaminyl tRNA synthetase right in the middle. |
1EXD.pdb
|
|
