Difference between revisions of "BIN-SX-Small molecules"
m |
m |
||
| Line 19: | Line 19: | ||
| − | {{ | + | {{LIVE}} |
{{Vspace}} | {{Vspace}} | ||
| Line 29: | Line 29: | ||
<section begin=abstract /> | <section begin=abstract /> | ||
<!-- included from "../components/BIN-SX-Small_molecules.components.wtxt", section: "abstract" --> | <!-- included from "../components/BIN-SX-Small_molecules.components.wtxt", section: "abstract" --> | ||
| − | + | Creating with small molecule structures, finding complexes in the PDB that contain the molecule, and superimposing model and structure. | |
<section end=abstract /> | <section end=abstract /> | ||
| Line 51: | Line 51: | ||
=== Objectives === | === Objectives === | ||
<!-- included from "../components/BIN-SX-Small_molecules.components.wtxt", section: "objectives" --> | <!-- included from "../components/BIN-SX-Small_molecules.components.wtxt", section: "objectives" --> | ||
| − | ... | + | This unit will ... |
| + | * ... introduce options to model small molecules; | ||
| + | * ... demonstrate how to find PDB complexes that contain the molecule; | ||
| + | * ... teach how to superimpose model and structure; | ||
{{Vspace}} | {{Vspace}} | ||
| Line 58: | Line 61: | ||
=== Outcomes === | === Outcomes === | ||
<!-- included from "../components/BIN-SX-Small_molecules.components.wtxt", section: "outcomes" --> | <!-- included from "../components/BIN-SX-Small_molecules.components.wtxt", section: "outcomes" --> | ||
| − | ... | + | After working through this unit you ... |
| + | * ... can model small molecule structures and superimpose them onto cognate molecules in a protein structure ligand binding site. | ||
{{Vspace}} | {{Vspace}} | ||
| Line 77: | Line 81: | ||
=== Evaluation === | === Evaluation === | ||
<!-- included from "../components/BIN-SX-Small_molecules.components.wtxt", section: "evaluation" --> | <!-- included from "../components/BIN-SX-Small_molecules.components.wtxt", section: "evaluation" --> | ||
| − | <!-- included from "ABC-unit_components.wtxt", section: " | + | |
| − | < | + | This learning unit can be evaluated for a maximum of 6 marks. If you want to submit tasks for this unit for credit you have the following options. If you have any questions about these options, discuss on the mailing list. |
| − | : | + | |
| + | ;Short report option | ||
| + | :# Create a new page on the student Wiki as a subpage of your User Page. | ||
| + | :# Visit the [BIN-SX-Small_molecules_choices small-molecule selection page on the student wiki] and choose one small molecule to work with. | ||
| + | :# Summarize what this molecule is. Draw the structure of the molecule in one of the molecular editors described in the unit and produce a SMILES string. Find a suitable PDB complex structure and superimpose your model in Chimera. Report on the quality of the superposition and create an informative stereo image that illustrates how your model is situated in the ligand binding site relative to the experimentally determined compound. | ||
| + | :# When you are done with everything, add the following category tag to the page: | ||
| + | ::<code><nowiki>[[Category:EVAL-BIN-SX-Small_molecules]]</nowiki></code> | ||
| + | :'''Do not''' change your submission page after this tag has been added. The page will be marked and the category tag will be removed by the instructor. | ||
| + | |||
| + | <!-- | ||
| + | ; Quiz option | ||
| + | : Open the [http://steipe.biochemistry.utoronto.ca/abc/students/index.php/Signup-BIN-PPI-Analysis_Quiz '''signup-page for the quiz for this unit (linked from here)'''] and add your name. Your name must be signed up by 12:00 of the day of the Quiz to ensure copies of the quiz are available for all participants. | ||
| + | <!-- included from "ABC-unit_components.wtxt", section: "quiz-mechanics" --> | ||
| + | :Quizzes will be written in class, back-to-back if there is more than one quiz scheduled. We may begin at any time. We will have an open-ended Q&A session before the quiz. You can't take the quiz if you are not present in class when the question sheets are handed out, so don't be late. Once all scheduled quizzes are written, we will discuss and mark them. You will mark your own quiz. All marking must be done with a red pen - so you '''must''' bring a red pen to class in order to participate. The mark you give yourself may be revised by the instructor after spot-checking quizzes. If this is necessary, you will be notified. You must mark your quiz correctly and honestly - don't get into trouble with academic integrity rules: it will be an academic offence if you mark questions as correct that were discussed in class and should have been marked incorrect. When in doubt, ask. | ||
| + | |||
| + | |||
| + | ; R-code option | ||
| + | :Submit code according to the following requirements. Make sure your code is documented. | ||
| + | |||
| + | |||
| + | ; Option to write a "Self-Evaluation Question" | ||
| + | :If you submit both [[BIN-NCBI]] and [[BIN-EBI]] for evaluation, you can choose this option for only one of the two. | ||
| + | : Write a "Self-evaluation Question" (with a model solution) that explores a significant, non-trivial aspect of studying how to work with EBI resources within this learning unit. Ensure that the question is feasible, given the existing content of the unit - or coordinate an extension of the contents with your instructor. Ensure your question pursues a high-level learning goal, it should allow others to demonstrate understanding, critical analysis, and/or the capacity to integrate and synthesize knowledge, not merely test memorization. Ensure that your question is specific, not ambiguous, vague or tangential to the contents. Ensure you are testing '''valuable''' knowledge and skills, not Cargo Cult. Apply the [[ABC-Rubrics| '''marking rubrics''']] in spirit to satisfy yourself of the quality of your contribution. Obviously, details of evaluation will vary with the question. Use the format and code templates that you find on the [[Self_evaluation_questions|'''Self evaluation questions page''']] - but don't assume those examples are already models of excellent contributions. Note: assume that approximately the same amount of work is expected for all evaluation options. Consequently, the standard of excellence for this option will be quite high. | ||
| + | :#Create a new page on the student Wiki as a subpage of your User Page. Develop your question there. | ||
| + | :#When you are done with developing this contents, add the following category tag to the page: | ||
| + | ::<code><nowiki>[[Category:EVAL-BIN-NCBI]]</nowiki></code> | ||
| + | :'''Do not''' change your submission page after this tag has been added. The page will be marked and the category tag will be removed by the instructor. | ||
| + | --> | ||
{{Vspace}} | {{Vspace}} | ||
| Line 94: | Line 125: | ||
<!-- cf. Phil Fradkin's ChemoGenomics BCB410-2015 --> | <!-- cf. Phil Fradkin's ChemoGenomics BCB410-2015 --> | ||
| − | |||
{{Vspace}} | {{Vspace}} | ||
| − | |||
| − | |||
=== Modeling small molecules === | === Modeling small molecules === | ||
| Line 107: | Line 135: | ||
| − | {{ | + | {{Task|1= |
| + | ;Caffeine at PubChem | ||
# Access [http://pubchem.ncbi.nlm.nih.gov/ PubChem]. | # Access [http://pubchem.ncbi.nlm.nih.gov/ PubChem]. | ||
# Enter "caffeine" as a search term in the '''Compound''' tab. A number of matches to this keyword search are returned. | # Enter "caffeine" as a search term in the '''Compound''' tab. A number of matches to this keyword search are returned. | ||
| Line 113: | Line 142: | ||
## A 2D structural sketch; | ## A 2D structural sketch; | ||
## An idealized 3D structural conformer, for which you can download coordinates in several formats; | ## An idealized 3D structural conformer, for which you can download coordinates in several formats; | ||
| − | ## The IUPAC name: < | + | ## The IUPAC name: <tt>1,3,7-trimethylpurine-2,6-dione</tt>; |
## The CAS identifier <code>58-08-2</code> which is a unique identifier and can be used as a cross-reference ID; | ## The CAS identifier <code>58-08-2</code> which is a unique identifier and can be used as a cross-reference ID; | ||
## The {{WP|SMILES|SMILES strings|SMILES string}} <code>CN1C{{=}}NC2{{=}}C1C({{=}}O)N(C({{=}}O)N2C)C</code>; | ## The {{WP|SMILES|SMILES strings|SMILES string}} <code>CN1C{{=}}NC2{{=}}C1C({{=}}O)N(C({{=}}O)N2C)C</code>; | ||
| Line 120: | Line 149: | ||
| − | That's great, but let's sketch our own version of caffeine. Several versions of Peter Ertl's {{WP|JME_editor|Java Molecular Editor (JME)}} are offered online, PubChem offers this functionality via its '''Sketcher''' tool | + | {{Task|1= |
| + | ;Caffeine at DrugBank | ||
| + | # Access [https://www.drugbank.ca DrugBank]. | ||
| + | # Enter "Caffeine" in the search form and. . | ||
| + | # Click on the [https://www.drugbank.ca/drugs/DB00201 hit to "Caffeine" itself]. Note that the page contains among other items: | ||
| + | ## A detailed description | ||
| + | ## A 2D structural sketch with a link to 3D options; | ||
| + | ## Synonyms, including the IUPAC name: <tt>1,3,7-trimethylpurine-2,6-dione</tt>; | ||
| + | ## ... and much more. | ||
| + | # Follow the link [https://www.drugbank.ca/structures/small_molecule_drugs/DB00201 to the 3D options] and note the options for downloading information, including the SMILES string and PDB formatted coordinates. | ||
| + | }} | ||
| + | |||
| + | |||
| + | That's great, but let's sketch our own version of caffeine. Several versions of Peter Ertl's {{WP|JME_editor|Java Molecular Editor (JME)}} are offered online, PubChem offers this functionality via its '''Sketcher''' tool and the PDB has a similar sketching tool on its ligand search page] | ||
| − | {{ | + | {{Task|1= |
# Return to the [http://pubchem.ncbi.nlm.nih.gov/ PubChem homepage]. | # Return to the [http://pubchem.ncbi.nlm.nih.gov/ PubChem homepage]. | ||
# Follow the link to '''Structure search''' (in the right hand menu). | # Follow the link to '''Structure search''' (in the right hand menu). | ||
| Line 133: | Line 175: | ||
=== Translating SMILES to structure === | === Translating SMILES to structure === | ||
| − | Chimera can translate SMILES strings to coordinates<ref>There are several online servers that translate SMILES strings to idealized structures, | + | Chimera can translate SMILES strings to coordinates<ref>There are also several online servers that translate SMILES strings to idealized structures, for example the [http://cactus.nci.nih.gov/translate/ online SMILES translation service] at the NCI.</ref>. |
| − | {{ | + | {{Task|1= |
# Open Chimera. | # Open Chimera. | ||
# Select '''Tools''' → '''Structure Editing''' → '''Build Structure'''. | # Select '''Tools''' → '''Structure Editing''' → '''Build Structure'''. | ||
| Line 151: | Line 193: | ||
# Use the '''Actions''' → '''Surface''' → '''transparency''' → '''50%''' menu to see atoms and bonds that are covered by the surface. | # Use the '''Actions''' → '''Surface''' → '''transparency''' → '''50%''' menu to see atoms and bonds that are covered by the surface. | ||
# To begin working with molecules in "true" 3D, choose '''Tools''' → '''Viewing Controls''' → '''Camera''' and select '''camera mode''' → '''wall-eye stereo'''. Also, use the '''Effects''' tab of the '''Viewing''' window, and ''check'' '''shadows''' off. | # To begin working with molecules in "true" 3D, choose '''Tools''' → '''Viewing Controls''' → '''Camera''' and select '''camera mode''' → '''wall-eye stereo'''. Also, use the '''Effects''' tab of the '''Viewing''' window, and ''check'' '''shadows''' off. | ||
| − | # Your structure should look about like what you see below | + | # Your structure should look about like what you see below. |
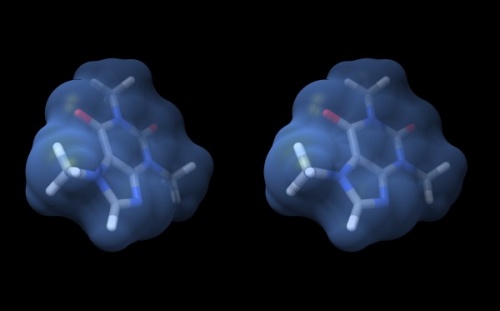
| − | {{ | + | {{Stereo|Caffeine_stereo.jpg|'''Wall-eye stereo view''' of the caffeine structure, surrounded by a transparent molecular surface. The image for the left eye is on the left side. |
}} | }} | ||
| Line 161: | Line 203: | ||
| − | {{ | + | ===Superposition=== |
| + | |||
| + | To investigate a small molecule structure variant in the context of a complex, we need to superimpose it with an existing ligand. | ||
| + | |||
| + | {{Task|1= | ||
| + | * Open the structure <tt>3G6M</tt> in Chimera. This is one of the hits returned from the PDB serach for caffeine - a fungal chitinase for which caffeine is a potent inhibitor. | ||
| + | * Choose '''Select''' → '''Residue''' → '''CFF''' (CFF is th PDB three-letter code for this hetero compound), then '''Select''' → '''Invert (selected models)''', '''Actions''' → '''Atoms/Bonds''' → '''hide''' and '''Actions''' → '''Ribbon''' → '''hide''' to show only the caffeine molecules - there are two. Select the one with residue ID 1, and again '''Actions''' → '''Atoms/Bonds''' → '''hide'''. The remaining CFF molecule has residue ID 427. | ||
| + | To superimpose the structures, we can't use the standard "match" option, because that only works for protein or DNA molecules. Instead, we need to explicitly define matching pairs of atoms through Chimera's command line interface. The command line interface is a very powerful way to issue Chimera commands, but it has a bit of a learning curve since we need to use a precise model/residue/atom selection syntax. | ||
| + | * Visit [http://www.rbvi.ucsf.edu/chimera/docs/UsersGuide/midas/atom_spec.html the Chimera Help page on atom specification]. Note how we specify models with a "#" sigil, residues with a ":" or "::" sigil, and atoms with an "@" sigil. | ||
| + | * Open the Chimera command line by clicking on the computer icon at the top left of the viewer window. | ||
| + | * The command we need is <tt>match</tt>, and we need to feed the command atoms in exactly the order of the pairs that the superposition algorithm should superimpose. To identify the atom numbers, we can hover over them with the mouse, or we can select the residue/atom and choose '''Actions''' → '''Label''' → '''name'''. If we superimpose the four nitrogen atoms, the correct command may be:<code>match #0@N3,N4,N1,N2 #1:427@N1,N3,N7,N9</code> to superimpose the model we built from the SMILES string onto the structure - but the exact atom names in the model structure depend on how the SMILES string was written. | ||
| + | * Note how the two structures virtually overlap - in this case, there are only very small coordinate differences because the conformational degrees of freedom are very much constrained in this xanthin hetrocycle. But there '''are''' differences nevertheless. One moleculae is an idealized structure, the other a structure that has been determined by a high-resolution experiment. | ||
| + | |||
| + | }} | ||
| + | |||
| + | {{Vspace}} | ||
| Line 234: | Line 291: | ||
:2017-08-05 | :2017-08-05 | ||
<b>Modified:</b><br /> | <b>Modified:</b><br /> | ||
| − | :2017- | + | :2017-11-10 |
<b>Version:</b><br /> | <b>Version:</b><br /> | ||
| − | :0 | + | :1.0 |
<b>Version history:</b><br /> | <b>Version history:</b><br /> | ||
| + | *1.0 First live version | ||
*0.1 First stub | *0.1 First stub | ||
</div> | </div> | ||
Revision as of 18:26, 10 November 2017
"Small Molecule" Structure"
Keywords: A small-molecule structure tutorial
Contents
Abstract
Creating with small molecule structures, finding complexes in the PDB that contain the molecule, and superimposing model and structure.
This unit ...
Prerequisites
You need the following preparation before beginning this unit. If you are not familiar with this material from courses you took previously, you need to prepare yourself from other information sources:
- Biomolecules: The molecules of life; nucleic acids and amino acids; the genetic code; protein folding; post-translational modifications and protein biochemistry; membrane proteins; biological function.
You need to complete the following units before beginning this one:
Objectives
This unit will ...
- ... introduce options to model small molecules;
- ... demonstrate how to find PDB complexes that contain the molecule;
- ... teach how to superimpose model and structure;
Outcomes
After working through this unit you ...
- ... can model small molecule structures and superimpose them onto cognate molecules in a protein structure ligand binding site.
Deliverables
- Time management: Before you begin, estimate how long it will take you to complete this unit. Then, record in your course journal: the number of hours you estimated, the number of hours you worked on the unit, and the amount of time that passed between start and completion of this unit.
- Journal: Document your progress in your Course Journal. Some tasks may ask you to include specific items in your journal. Don't overlook these.
- Insights: If you find something particularly noteworthy about this unit, make a note in your insights! page.
Evaluation
This learning unit can be evaluated for a maximum of 6 marks. If you want to submit tasks for this unit for credit you have the following options. If you have any questions about these options, discuss on the mailing list.
- Short report option
-
- Create a new page on the student Wiki as a subpage of your User Page.
- Visit the [BIN-SX-Small_molecules_choices small-molecule selection page on the student wiki] and choose one small molecule to work with.
- Summarize what this molecule is. Draw the structure of the molecule in one of the molecular editors described in the unit and produce a SMILES string. Find a suitable PDB complex structure and superimpose your model in Chimera. Report on the quality of the superposition and create an informative stereo image that illustrates how your model is situated in the ligand binding site relative to the experimentally determined compound.
- When you are done with everything, add the following category tag to the page:
[[Category:EVAL-BIN-SX-Small_molecules]]
- Do not change your submission page after this tag has been added. The page will be marked and the category tag will be removed by the instructor.
- Quizzes will be written in class, back-to-back if there is more than one quiz scheduled. We may begin at any time. We will have an open-ended Q&A session before the quiz. You can't take the quiz if you are not present in class when the question sheets are handed out, so don't be late. Once all scheduled quizzes are written, we will discuss and mark them. You will mark your own quiz. All marking must be done with a red pen - so you must bring a red pen to class in order to participate. The mark you give yourself may be revised by the instructor after spot-checking quizzes. If this is necessary, you will be notified. You must mark your quiz correctly and honestly - don't get into trouble with academic integrity rules: it will be an academic offence if you mark questions as correct that were discussed in class and should have been marked incorrect. When in doubt, ask.
- R-code option
- Submit code according to the following requirements. Make sure your code is documented.
- Option to write a "Self-Evaluation Question"
- If you submit both BIN-NCBI and BIN-EBI for evaluation, you can choose this option for only one of the two.
- Write a "Self-evaluation Question" (with a model solution) that explores a significant, non-trivial aspect of studying how to work with EBI resources within this learning unit. Ensure that the question is feasible, given the existing content of the unit - or coordinate an extension of the contents with your instructor. Ensure your question pursues a high-level learning goal, it should allow others to demonstrate understanding, critical analysis, and/or the capacity to integrate and synthesize knowledge, not merely test memorization. Ensure that your question is specific, not ambiguous, vague or tangential to the contents. Ensure you are testing valuable knowledge and skills, not Cargo Cult. Apply the marking rubrics in spirit to satisfy yourself of the quality of your contribution. Obviously, details of evaluation will vary with the question. Use the format and code templates that you find on the Self evaluation questions page - but don't assume those examples are already models of excellent contributions. Note: assume that approximately the same amount of work is expected for all evaluation options. Consequently, the standard of excellence for this option will be quite high.
- Create a new page on the student Wiki as a subpage of your User Page. Develop your question there.
- When you are done with developing this contents, add the following category tag to the page:
[[Category:EVAL-BIN-NCBI]]
- Do not change your submission page after this tag has been added. The page will be marked and the category tag will be removed by the instructor.
-->
Contents
Task:
- Read the introductory notes on working with "small molecule" structure.
Modeling small molecules
"Small" molecules are solvent, ligands, substrates, products, prosthetic groups, drugs - in short, essentially everything that is not made by DNA-, RNA-polymerases or the ribosome. Whereas the biopolymers are still front and centre in our quest to understand molecular biology, small molecules are crucial for our quest to interact with the inventory of the cell, create useful products, or advance medicine.
A number of public repositories make small-molecule information available, such as PubChem at the NCBI, the ligand collection at the PDB, the ChEBI database at the European Bioinformatics Institute, the Canadian DrugBank, or the NCI database browser at the US National Cancer Institute. One general way to export topology information from these services is to use SMILES strings—a shorthand notation for the composition and topology of chemical compounds.
Task:
- Caffeine at PubChem
- Access PubChem.
- Enter "caffeine" as a search term in the Compound tab. A number of matches to this keyword search are returned.
- Click on the top hit - 1,3,7-Trimethylxanthine, the Caffeine molecule. Note that the page contains among other items:
- A 2D structural sketch;
- An idealized 3D structural conformer, for which you can download coordinates in several formats;
- The IUPAC name: 1,3,7-trimethylpurine-2,6-dione;
- The CAS identifier
58-08-2which is a unique identifier and can be used as a cross-reference ID; - The SMILES strings
CN1C=NC2=C1C(=O)N(C(=O)N2C)C; - ... and much more.
Task:
- Caffeine at DrugBank
- Access DrugBank.
- Enter "Caffeine" in the search form and. .
- Click on the hit to "Caffeine" itself. Note that the page contains among other items:
- A detailed description
- A 2D structural sketch with a link to 3D options;
- Synonyms, including the IUPAC name: 1,3,7-trimethylpurine-2,6-dione;
- ... and much more.
- Follow the link to the 3D options and note the options for downloading information, including the SMILES string and PDB formatted coordinates.
That's great, but let's sketch our own version of caffeine. Several versions of Peter Ertl's Java Molecular Editor (JME) are offered online, PubChem offers this functionality via its Sketcher tool and the PDB has a similar sketching tool on its ligand search page]
Task:
- Return to the PubChem homepage.
- Follow the link to Structure search (in the right hand menu).
- Click on the 3D conformer tab and on the Launch button to launch the molecular editor in its own window.
- Sketch the structure of caffeine. I find the editor quite intuitive but clicking on the Help button will give you a quick, structured overview. Make sure you define your double-bonds correctly.
- Export the SMILES string of your compound to your project folder.
Translating SMILES to structure
Chimera can translate SMILES strings to coordinates[1].
Task:
- Open Chimera.
- Select Tools → Structure Editing → Build Structure.
- In the Build Structure window, select the SMILES string button, paste the string from your file, and click Apply.
- The caffeine molecule will be generated and visualized in the graphics window. This is a "stick" representation.
- You can rotate it with your mouse, <command> drag to scale, <shift> drag to translate.
- Use the Actions → Atoms/Bonds → ball & stick or sphere menu items to change appearance.
- Use the Actions → Color → by element menu to change colors.
- Change the display back to stick and use Actions → Surface → show to add a solvent accessible surface. Choosing this command triggers the calculation of the surface, which is then available as an individually selectable object. However, with default parameters the surface appears a bit rough for this small molecule.
- Change the parameters of this solvent accessible surface:
- Select the surface with <control><click> (<control><left mouse button> on windows). A green contour line appears around selected items – it surrounds the surface in this case.
- Open the selection inspector by clicking on the tiny green icon in the lower-right corner of the window (It has a magnifying glass symbol which means "inspect" for Chimera, not "search").
- Select Inspect ...MSMS surface and change the Vertex density value to 50.0 - hit return.
- By default, the surface inherits the colour of the atoms it envelopes. To change the colour of the surface, use the Actions → Color → all options menu. Click the surfaces button to indicate that the color choice should be applied to the surface object (note what else you can apply color to...), then choose cornflower blue.
- Use the Actions → Surface → transparency → 50% menu to see atoms and bonds that are covered by the surface.
- To begin working with molecules in "true" 3D, choose Tools → Viewing Controls → Camera and select camera mode → wall-eye stereo. Also, use the Effects tab of the Viewing window, and check shadows off.
- Your structure should look about like what you see below.
Superposition
To investigate a small molecule structure variant in the context of a complex, we need to superimpose it with an existing ligand.
Task:
- Open the structure 3G6M in Chimera. This is one of the hits returned from the PDB serach for caffeine - a fungal chitinase for which caffeine is a potent inhibitor.
- Choose Select → Residue → CFF (CFF is th PDB three-letter code for this hetero compound), then Select → Invert (selected models), Actions → Atoms/Bonds → hide and Actions → Ribbon → hide to show only the caffeine molecules - there are two. Select the one with residue ID 1, and again Actions → Atoms/Bonds → hide. The remaining CFF molecule has residue ID 427.
To superimpose the structures, we can't use the standard "match" option, because that only works for protein or DNA molecules. Instead, we need to explicitly define matching pairs of atoms through Chimera's command line interface. The command line interface is a very powerful way to issue Chimera commands, but it has a bit of a learning curve since we need to use a precise model/residue/atom selection syntax.
- Visit the Chimera Help page on atom specification. Note how we specify models with a "#" sigil, residues with a ":" or "::" sigil, and atoms with an "@" sigil.
- Open the Chimera command line by clicking on the computer icon at the top left of the viewer window.
- The command we need is match, and we need to feed the command atoms in exactly the order of the pairs that the superposition algorithm should superimpose. To identify the atom numbers, we can hover over them with the mouse, or we can select the residue/atom and choose Actions → Label → name. If we superimpose the four nitrogen atoms, the correct command may be:
match #0@N3,N4,N1,N2 #1:427@N1,N3,N7,N9to superimpose the model we built from the SMILES string onto the structure - but the exact atom names in the model structure depend on how the SMILES string was written. - Note how the two structures virtually overlap - in this case, there are only very small coordinate differences because the conformational degrees of freedom are very much constrained in this xanthin hetrocycle. But there are differences nevertheless. One moleculae is an idealized structure, the other a structure that has been determined by a high-resolution experiment.
Further reading, links and resources
Notes
- ↑ There are also several online servers that translate SMILES strings to idealized structures, for example the online SMILES translation service at the NCI.
Self-evaluation
If in doubt, ask! If anything about this learning unit is not clear to you, do not proceed blindly but ask for clarification. Post your question on the course mailing list: others are likely to have similar problems. Or send an email to your instructor.
About ...
Author:
- Boris Steipe <boris.steipe@utoronto.ca>
Created:
- 2017-08-05
Modified:
- 2017-11-10
Version:
- 1.0
Version history:
- 1.0 First live version
- 0.1 First stub
![]() This copyrighted material is licensed under a Creative Commons Attribution 4.0 International License. Follow the link to learn more.
This copyrighted material is licensed under a Creative Commons Attribution 4.0 International License. Follow the link to learn more.