Difference between revisions of "BIN-SX-Small molecules"
m (Created page with "<div id="BIO"> <div class="b1"> "Small Molecule" Structure" </div> {{Vspace}} <div class="keywords"> <b>Keywords:</b> A small-molecule structure tutorial </div...") |
m |
||
| Line 19: | Line 19: | ||
| − | {{ | + | {{DEV}} |
{{Vspace}} | {{Vspace}} | ||
| Line 42: | Line 42: | ||
<!-- included from "ABC-unit_components.wtxt", section: "notes-prerequisites" --> | <!-- included from "ABC-unit_components.wtxt", section: "notes-prerequisites" --> | ||
You need to complete the following units before beginning this one: | You need to complete the following units before beginning this one: | ||
| − | *[[BIN- | + | *[[BIN-SX-Chimera]] |
{{Vspace}} | {{Vspace}} | ||
| Line 86: | Line 86: | ||
== Contents == | == Contents == | ||
<!-- included from "../components/BIN-SX-Small_molecules.components.wtxt", section: "contents" --> | <!-- included from "../components/BIN-SX-Small_molecules.components.wtxt", section: "contents" --> | ||
| − | ... | + | |
| + | {{Task|1= | ||
| + | *Read the introductory notes on {{ABC-PDF|BIN-SX-Small_molecules|working with "small molecule" structure}}. | ||
| + | }} | ||
| + | |||
| + | |||
| + | {{Vspace}} | ||
| + | |||
| + | |||
| + | |||
| + | === Modeling small molecules === | ||
| + | |||
| + | "Small" molecules are solvent, ligands, substrates, products, prosthetic groups, drugs - in short, essentially everything that is not made by DNA-, RNA-polymerases or the ribosome. Whereas the biopolymers are still front and centre in our quest to understand molecular biology, small molecules are crucial for our quest to interact with the inventory of the cell, create useful products, or advance medicine. | ||
| + | |||
| + | A number of public repositories make small-molecule information available, such as [http://pubchem.ncbi.nlm.nih.gov/ PubChem] at the NCBI, the ligand collection at the [http://pdb.org '''PDB'''], the [http://www.ebi.ac.uk/chebi/ ChEBI] database at the European Bioinformatics Institute, the Canadian [http://www.drugbank.ca DrugBank], or the [http://cactus.nci.nih.gov/ncidb2.2/ NCI database browser] at the US National Cancer Institute. One general way to export topology information from these services is to use {{WP|SMILES|SMILES strings}}—a shorthand notation for the composition and topology of chemical compounds. | ||
| + | |||
| + | |||
| + | {{task| | ||
| + | # Access [http://pubchem.ncbi.nlm.nih.gov/ PubChem]. | ||
| + | # Enter "caffeine" as a search term in the '''Compound''' tab. A number of matches to this keyword search are returned. | ||
| + | # Click on the [http://pubchem.ncbi.nlm.nih.gov/compound/2519 top hit - 1,3,7-Trimethylxanthine, the Caffeine molecule]. Note that the page contains among other items: | ||
| + | ## A 2D structural sketch; | ||
| + | ## An idealized 3D structural conformer, for which you can download coordinates in several formats; | ||
| + | ## The IUPAC name: <code>1,3,7-trimethylpurine-2,6-dione</code>; | ||
| + | ## The CAS identifier <code>58-08-2</code> which is a unique identifier and can be used as a cross-reference ID; | ||
| + | ## The {{WP|SMILES|SMILES strings|SMILES string}} <code>CN1C{{=}}NC2{{=}}C1C({{=}}O)N(C({{=}}O)N2C)C</code>; | ||
| + | ## ... and much more. | ||
| + | }} | ||
| + | |||
| + | |||
| + | That's great, but let's sketch our own version of caffeine. Several versions of Peter Ertl's {{WP|JME_editor|Java Molecular Editor (JME)}} are offered online, PubChem offers this functionality via its '''Sketcher''' tool. | ||
| + | |||
| + | {{task| | ||
| + | # Return to the [http://pubchem.ncbi.nlm.nih.gov/ PubChem homepage]. | ||
| + | # Follow the link to '''Structure search''' (in the right hand menu). | ||
| + | # Click on the '''3D conformer''' tab and on the '''Launch''' button to launch the molecular editor in its own window. | ||
| + | # Sketch the structure of caffeine. I find the editor quite intuitive but clicking on the '''Help''' button will give you a quick, structured overview. Make sure you define your double-bonds correctly. | ||
| + | # '''Export''' the SMILES string of your compound to your project folder. | ||
| + | }} | ||
| + | |||
| + | |||
| + | === Translating SMILES to structure === | ||
| + | |||
| + | Chimera can translate SMILES strings to coordinates<ref>There are several online servers that translate SMILES strings to idealized structures, see e.g. the [http://cactus.nci.nih.gov/translate/ online SMILES translation service] at the NCI.</ref>. | ||
| + | |||
| + | {{task| | ||
| + | # Open Chimera. | ||
| + | # Select '''Tools''' → '''Structure Editing''' → '''Build Structure'''. | ||
| + | # In the '''Build Structure''' window, select the '''SMILES string''' button, paste the string from your file, and click '''Apply'''. | ||
| + | # The caffeine molecule will be generated and visualized in the graphics window. This is a "stick" representation. | ||
| + | # You can rotate it with your mouse, <command> drag to scale, <shift> drag to translate. | ||
| + | # Use the '''Actions''' → '''Atoms/Bonds''' → '''ball & stick''' or '''sphere''' menu items to change appearance. | ||
| + | # Use the '''Actions''' → '''Color''' → '''by element''' menu to change colors. | ||
| + | # Change the display back to stick and use '''Actions''' → '''Surface''' → '''show''' to add a solvent accessible surface. Choosing this command triggers the calculation of the surface, which is then available as an individually selectable object. However, with default parameters the surface appears a bit rough for this small molecule. | ||
| + | # Change the parameters of this solvent accessible surface: | ||
| + | ## Select the surface with <control><click> (<control><left mouse button> on windows). A green contour line appears around selected items – it surrounds the surface in this case. | ||
| + | ## Open the selection inspector by clicking on the tiny green icon in the lower-right corner of the window (It has a magnifying glass symbol which means "inspect" for Chimera, not "search"). | ||
| + | ## Select Inspect ...'''MSMS surface''' and change the '''Vertex density''' value to 50.0 - hit return. | ||
| + | # By default, the surface inherits the colour of the atoms it envelopes. To change the colour of the surface, use the '''Actions''' → '''Color''' → '''all options''' menu. Click the '''surfaces''' button to indicate that the color choice should be applied to the surface object (note what else you can apply color to...), then choose '''cornflower blue'''. | ||
| + | # Use the '''Actions''' → '''Surface''' → '''transparency''' → '''50%''' menu to see atoms and bonds that are covered by the surface. | ||
| + | # To begin working with molecules in "true" 3D, choose '''Tools''' → '''Viewing Controls''' → '''Camera''' and select '''camera mode''' → '''wall-eye stereo'''. Also, use the '''Effects''' tab of the '''Viewing''' window, and ''check'' '''shadows''' off. | ||
| + | # Your structure should look about like what you see below. Save your session with the '''File''' → '''Save Session''' dialogue so you can easily recreate the scene. | ||
| + | |||
| + | |||
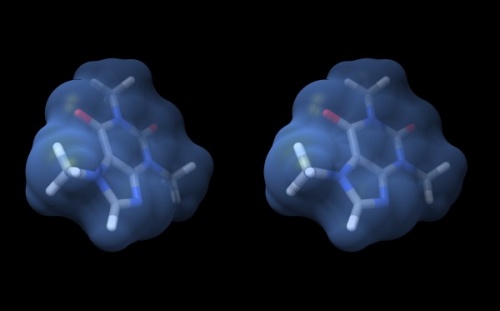
| + | {{stereo|Caffeine_stereo.jpg|'''Wall-eye stereo view''' of the caffeine structure, surrounded by a transparent molecular surface. The image for the left eye is on the left side. For instructions on ''stereo-viewing'', see the next section. | ||
| + | }} | ||
| + | |||
| + | |||
| + | }} | ||
| + | |||
| + | |||
| + | {{Vspace}} | ||
| + | |||
| + | |||
| + | |||
{{Vspace}} | {{Vspace}} | ||
Revision as of 04:31, 31 August 2017
"Small Molecule" Structure"
Keywords: A small-molecule structure tutorial
Contents
This unit is under development. There is some contents here but it is incomplete and/or may change significantly: links may lead to nowhere, the contents is likely going to be rearranged, and objectives, deliverables etc. may be incomplete or missing. Do not work with this material until it is updated to "live" status.
Abstract
...
This unit ...
Prerequisites
You need the following preparation before beginning this unit. If you are not familiar with this material from courses you took previously, you need to prepare yourself from other information sources:
- Biomolecules: The molecules of life; nucleic acids and amino acids; the genetic code; protein folding; post-translational modifications and protein biochemistry; membrane proteins; biological function.
You need to complete the following units before beginning this one:
Objectives
...
Outcomes
...
Deliverables
- Time management: Before you begin, estimate how long it will take you to complete this unit. Then, record in your course journal: the number of hours you estimated, the number of hours you worked on the unit, and the amount of time that passed between start and completion of this unit.
- Journal: Document your progress in your course journal.
- Insights: If you find something particularly noteworthy about this unit, make a note in your insights! page.
Evaluation
Evaluation: NA
- This unit is not evaluated for course marks.
Contents
Task:
- Read the introductory notes on working with "small molecule" structure.
Modeling small molecules
"Small" molecules are solvent, ligands, substrates, products, prosthetic groups, drugs - in short, essentially everything that is not made by DNA-, RNA-polymerases or the ribosome. Whereas the biopolymers are still front and centre in our quest to understand molecular biology, small molecules are crucial for our quest to interact with the inventory of the cell, create useful products, or advance medicine.
A number of public repositories make small-molecule information available, such as PubChem at the NCBI, the ligand collection at the PDB, the ChEBI database at the European Bioinformatics Institute, the Canadian DrugBank, or the NCI database browser at the US National Cancer Institute. One general way to export topology information from these services is to use SMILES strings—a shorthand notation for the composition and topology of chemical compounds.
Task:
- Access PubChem.
- Enter "caffeine" as a search term in the Compound tab. A number of matches to this keyword search are returned.
- Click on the top hit - 1,3,7-Trimethylxanthine, the Caffeine molecule. Note that the page contains among other items:
- A 2D structural sketch;
- An idealized 3D structural conformer, for which you can download coordinates in several formats;
- The IUPAC name:
1,3,7-trimethylpurine-2,6-dione; - The CAS identifier
58-08-2which is a unique identifier and can be used as a cross-reference ID; - The SMILES strings
CN1C=NC2=C1C(=O)N(C(=O)N2C)C; - ... and much more.
That's great, but let's sketch our own version of caffeine. Several versions of Peter Ertl's Java Molecular Editor (JME) are offered online, PubChem offers this functionality via its Sketcher tool.
Task:
- Return to the PubChem homepage.
- Follow the link to Structure search (in the right hand menu).
- Click on the 3D conformer tab and on the Launch button to launch the molecular editor in its own window.
- Sketch the structure of caffeine. I find the editor quite intuitive but clicking on the Help button will give you a quick, structured overview. Make sure you define your double-bonds correctly.
- Export the SMILES string of your compound to your project folder.
Translating SMILES to structure
Chimera can translate SMILES strings to coordinates[1].
Task:
- Open Chimera.
- Select Tools → Structure Editing → Build Structure.
- In the Build Structure window, select the SMILES string button, paste the string from your file, and click Apply.
- The caffeine molecule will be generated and visualized in the graphics window. This is a "stick" representation.
- You can rotate it with your mouse, <command> drag to scale, <shift> drag to translate.
- Use the Actions → Atoms/Bonds → ball & stick or sphere menu items to change appearance.
- Use the Actions → Color → by element menu to change colors.
- Change the display back to stick and use Actions → Surface → show to add a solvent accessible surface. Choosing this command triggers the calculation of the surface, which is then available as an individually selectable object. However, with default parameters the surface appears a bit rough for this small molecule.
- Change the parameters of this solvent accessible surface:
- Select the surface with <control><click> (<control><left mouse button> on windows). A green contour line appears around selected items – it surrounds the surface in this case.
- Open the selection inspector by clicking on the tiny green icon in the lower-right corner of the window (It has a magnifying glass symbol which means "inspect" for Chimera, not "search").
- Select Inspect ...MSMS surface and change the Vertex density value to 50.0 - hit return.
- By default, the surface inherits the colour of the atoms it envelopes. To change the colour of the surface, use the Actions → Color → all options menu. Click the surfaces button to indicate that the color choice should be applied to the surface object (note what else you can apply color to...), then choose cornflower blue.
- Use the Actions → Surface → transparency → 50% menu to see atoms and bonds that are covered by the surface.
- To begin working with molecules in "true" 3D, choose Tools → Viewing Controls → Camera and select camera mode → wall-eye stereo. Also, use the Effects tab of the Viewing window, and check shadows off.
- Your structure should look about like what you see below. Save your session with the File → Save Session dialogue so you can easily recreate the scene.
Wall-eye stereo view of the caffeine structure, surrounded by a transparent molecular surface. The image for the left eye is on the left side. For instructions on stereo-viewing, see the next section.
Further reading, links and resources
Notes
- ↑ There are several online servers that translate SMILES strings to idealized structures, see e.g. the online SMILES translation service at the NCI.
Self-evaluation
If in doubt, ask! If anything about this learning unit is not clear to you, do not proceed blindly but ask for clarification. Post your question on the course mailing list: others are likely to have similar problems. Or send an email to your instructor.
About ...
Author:
- Boris Steipe <boris.steipe@utoronto.ca>
Created:
- 2017-08-05
Modified:
- 2017-08-05
Version:
- 0.1
Version history:
- 0.1 First stub
![]() This copyrighted material is licensed under a Creative Commons Attribution 4.0 International License. Follow the link to learn more.
This copyrighted material is licensed under a Creative Commons Attribution 4.0 International License. Follow the link to learn more.