Difference between revisions of "Stereo Vision Exam Questions"
(→2004) |
(→2005) |
||
| Line 145: | Line 145: | ||
<br> | <br> | ||
<br> | <br> | ||
| + | |||
| + | ==2006== | ||
| + | <br> | ||
| + | |||
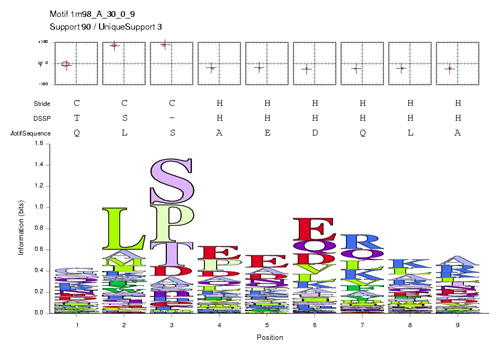
| + | In order to study structural and functional conservation patterns in the Mbp1 recognition helix, you obtain a structural motif analysis from a collaborator. The segment of residues 44 to 53 (numbering follows the 1MB1 structure) corresponds to a well defined helix N-cap motif. This motif is a structural pattern that recurrs 90 times in the Nh3D database of non-homologous proteins. The amino acid propensities of this pattern are summarized in the sequence-logo below: | ||
| + | |||
| + | <br> | ||
| + | |||
| + | [[Image:MotifSequenceLogoSmall.jpg|frame|none|Sequence logo for a nine-amino acid helix N-cap motif (1m98_A_30_0_9). ]] | ||
| + | |||
| + | <br> | ||
| + | |||
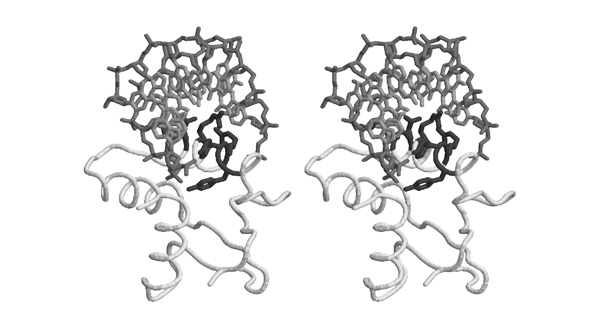
| + | After loading the PDB coordinate file 1MB1, a stereo view was generated with the following RasMol commands: | ||
| + | |||
| + | set background white | ||
| + | set stereo -5 | ||
| + | restrict 44-53 | ||
| + | color white | ||
| + | wireframe 80 | ||
| + | select backbone | ||
| + | color [170,170,170] | ||
| + | select 46 | ||
| + | color [80,80,80] | ||
| + | select 53-60 and backbone | ||
| + | wireframe 80 | ||
| + | select 6 and HOH | ||
| + | cpk 100 | ||
| + | |||
| + | <br> | ||
| + | [[Image:Helix_N-cap.jpg|frame|none|Divergent stereo view of residues 44 to 53 of 1MB1 as well as the backbone atoms of residues 54 to 60. The view is from the solvent-accessible surface towards the core of the protein, it includes the putative recognition helix of Mbp1, the 3 residues leading up to it, as well as a water molecule from the solvent shell. This structured water molecule is observed in approximately the location that a sidechain hydroxyl in position 46 would occupy. To facilitate orientation, Ala46 has been colored dark; it corresponds to residue 3 of the N-cap motif sequence logo given above. ]] | ||
| + | |||
| + | <br> | ||
| + | <div style="padding: 5px; background: #DDDDDD; border:solid 1px #000000;"> | ||
| + | *'''Briefly explain the high propensity for S/T in position 3 of the N-cap motif. This corresponds to Ala46 of 1MB1, which is not highly conserved (cf. question 1).''' | ||
| + | *'''Briefly explain the high propensity for R in position 7 of the N-cap motif . This corresponds to Arg50 of 1MB1, which is highly conserved (cf. question 1). Is this an example of structural or functional conservation?''' | ||
| + | </div> | ||
| + | <br> | ||
| + | <br> | ||
| + | |||
Revision as of 19:51, 18 October 2007
- Molecules are three-dimensional entities and stereo-vision of images on paper and on the screen is one of the most powerful, intuitive ways to appreciate that. We have practiced stero-vision in this course; here are a number of situations that require spatial awareness.
2002
- Write down the label of the tryptophan that is a conserved element of the hydrophobic core of this domain.
2002
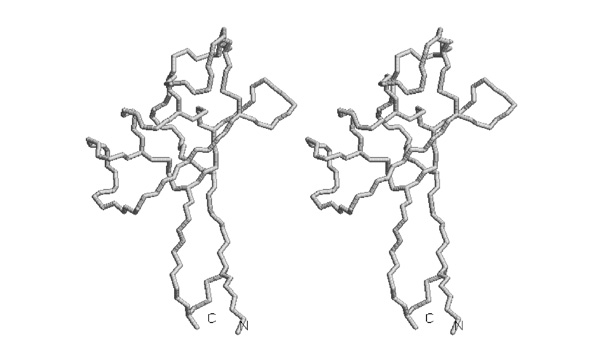
- Trace the backbone from the N-terminus to amino acid 52 with pencil or pen in one of the images.
I wouldn't ask this type of question any longer - the drawing task seems a bit convoluted for the actual skill it is supposed to test.
2003
As you know, a strong dipole moment is generated from the synergistic interactions of carbonyl groups in alpha helices. The carbonyls point towards the negative potential.
- Which three alpha helices are oriented best, so that their helix-dipole moment is aligned for favourable interactions with the ATP phosphate groups?
2003
The figure was generated with the following RasMol commands:
set background white set stereo -5 select all color white restrict helix and backbone wireframe 90 select glu,asp color [80,80,80]
- Mark on this sheet the position of those Asp or Glu residues that are positioned to interact favourably with the helix dipole. If there are several plausible residues in a helix, mark the one closest to the correct terminus.
2003
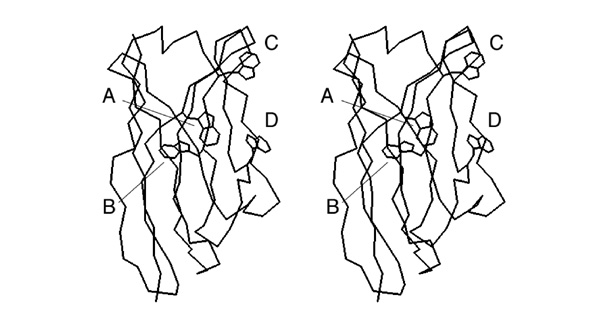
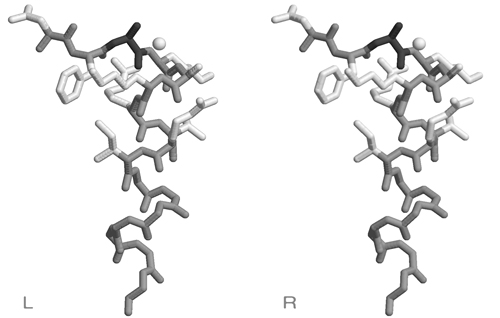
- Trace the disulfide bonded cysteine sidechains in one of the stereoviews of this picture.
2004
- Number the cysteines from 1 to 8, from N- to C- terminus and determine the disulfide bonding topology of this protein. Write the disulfide bonded residue pairs into your exam booklet.
2004
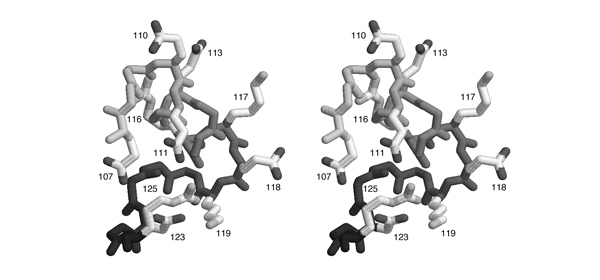
- List all pairs of residues that form salt-bridges according to this figure. Write the one-letter code for the negatively charged amino acid and its sequence number, then the same for the positively charged amino acid.
Comment
2004

HIGH" region is included here, its sequence is HVGT in this case. Carbons are shown in light grey, nitrogen atoms are darker, oxygen atoms are darkest grey and sulphur and phosphorous are black.
- Based on this figure, argue briefly why "G" is a conserved residue in the "
HIGH" motif.
2005
Base specific recognition of canonically paired nucleotides can be mediated through specific recognition sites that contribute H-bond donors/acceptors or methyl groups. One example of such recognition sites for a particular basepair is sketched here:
The sketch corresponds to the situation in the stereo figure below. It was generated with the following RasMol commands to demonstrate the fold of the domain and several important sidechains on the recognition helix that interact with various parts of the DNA.
set background white select all wireframe off restrict *c color white wireframe off trace 90 select (58,59,62,65-67) and *c select (*.ca or sidechain) and selected color [80,80,80] wireframe 100 select (5-11 and *a) or (3-9 and *b) color [190,190,190] wireframe 80 select (*a or *b) and backbone color [140,140,140] set stereo -5
- Identify the "C" and "D" recognition interactions depicted in the sketch above. Note the one-letter code(s) for the amino acid type(s) that is/are involved in these interactions.
2006
In order to study structural and functional conservation patterns in the Mbp1 recognition helix, you obtain a structural motif analysis from a collaborator. The segment of residues 44 to 53 (numbering follows the 1MB1 structure) corresponds to a well defined helix N-cap motif. This motif is a structural pattern that recurrs 90 times in the Nh3D database of non-homologous proteins. The amino acid propensities of this pattern are summarized in the sequence-logo below:
After loading the PDB coordinate file 1MB1, a stereo view was generated with the following RasMol commands:
set background white set stereo -5 restrict 44-53 color white wireframe 80 select backbone color [170,170,170] select 46 color [80,80,80] select 53-60 and backbone wireframe 80 select 6 and HOH cpk 100
- Briefly explain the high propensity for S/T in position 3 of the N-cap motif. This corresponds to Ala46 of 1MB1, which is not highly conserved (cf. question 1).
- Briefly explain the high propensity for R in position 7 of the N-cap motif . This corresponds to Arg50 of 1MB1, which is highly conserved (cf. question 1). Is this an example of structural or functional conservation?