Difference between revisions of "Aminoacid tutorial"
Jump to navigation
Jump to search
m (→Contents) |
m (→Contents) |
||
| (One intermediate revision by the same user not shown) | |||
| Line 14: | Line 14: | ||
--> | --> | ||
| − | This page contains | + | This page contains essential information on the 20 proteinogenic amino acids that every bioinformatician should commit to memory. |
| + | |||
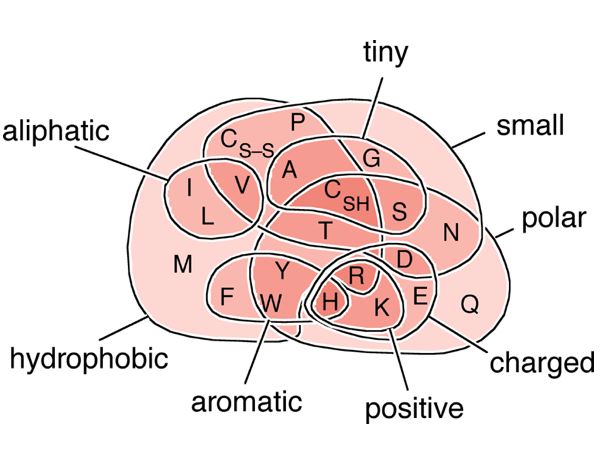
| + | [[Image:AA-Venn.jpg|frame|none|A Venn diagram of biophysical amino acid properties.<br> | ||
| + | <!-- caption -->The 20 proteinogenic amino acids are represented by their one letter code and grouped according to key biophysical properties. Cysteine appears twice: Once as '''C<sub>SH</sub>''', ''i.e.'' cysteine proper, a rather hydrophilic amino acid, isosteric to serine, with a free thiol group. The other is '''C<sub>S–S</sub>''', "cystine", the disulfide product of the oxidation of two cysteines. Complementary categories are implied in the diagram - e.g. those that are not ''small'' are ''large'', those that are ''charged'' but not ''positively charged'' are ''negatively charged'', those that are not ''hydrophobic'' are ''hydrophilic''. | ||
| + | ]] | ||
| Line 23: | Line 27: | ||
==Contents== | ==Contents== | ||
| − | + | What properties of the 20 proteinogenic amino acids are worthwhile to remember? Obviously, all those that would be inconvenient to look up when we need them. In practice, this means all those properties that allow us to "read" sequences and evaluate pairwise and multiple sequence alignments. These properties include: | |
| + | * relative size and shape | ||
| + | * relative hydrophobicity | ||
| + | * charge | ||
| + | * key properties of functionally important side chains | ||
| + | * H-bond donor and acceptor functions | ||
| + | * relative abundance | ||
| + | * propensity of amino acids for secondary structure | ||
| + | |||
| + | |||
| + | The contents for this page needs to be reconstructed, please see {{WP|Proteinogenic amino acid|'''the Wikipedia page on proteinogenic amino acids'''}} in the interim. | ||
Latest revision as of 22:14, 28 October 2013
The Aminoacids
This page is a placeholder, or under current development; it is here principally to establish the logical framework of the site. The material on this page is correct, but incomplete.
This page contains essential information on the 20 proteinogenic amino acids that every bioinformatician should commit to memory.

A Venn diagram of biophysical amino acid properties.
The 20 proteinogenic amino acids are represented by their one letter code and grouped according to key biophysical properties. Cysteine appears twice: Once as CSH, i.e. cysteine proper, a rather hydrophilic amino acid, isosteric to serine, with a free thiol group. The other is CS–S, "cystine", the disulfide product of the oxidation of two cysteines. Complementary categories are implied in the diagram - e.g. those that are not small are large, those that are charged but not positively charged are negatively charged, those that are not hydrophobic are hydrophilic.
The 20 proteinogenic amino acids are represented by their one letter code and grouped according to key biophysical properties. Cysteine appears twice: Once as CSH, i.e. cysteine proper, a rather hydrophilic amino acid, isosteric to serine, with a free thiol group. The other is CS–S, "cystine", the disulfide product of the oxidation of two cysteines. Complementary categories are implied in the diagram - e.g. those that are not small are large, those that are charged but not positively charged are negatively charged, those that are not hydrophobic are hydrophilic.
Contents
What properties of the 20 proteinogenic amino acids are worthwhile to remember? Obviously, all those that would be inconvenient to look up when we need them. In practice, this means all those properties that allow us to "read" sequences and evaluate pairwise and multiple sequence alignments. These properties include:
- relative size and shape
- relative hydrophobicity
- charge
- key properties of functionally important side chains
- H-bond donor and acceptor functions
- relative abundance
- propensity of amino acids for secondary structure
The contents for this page needs to be reconstructed, please see the Wikipedia page on proteinogenic amino acids in the interim.
Further reading and resources